Life Sciences
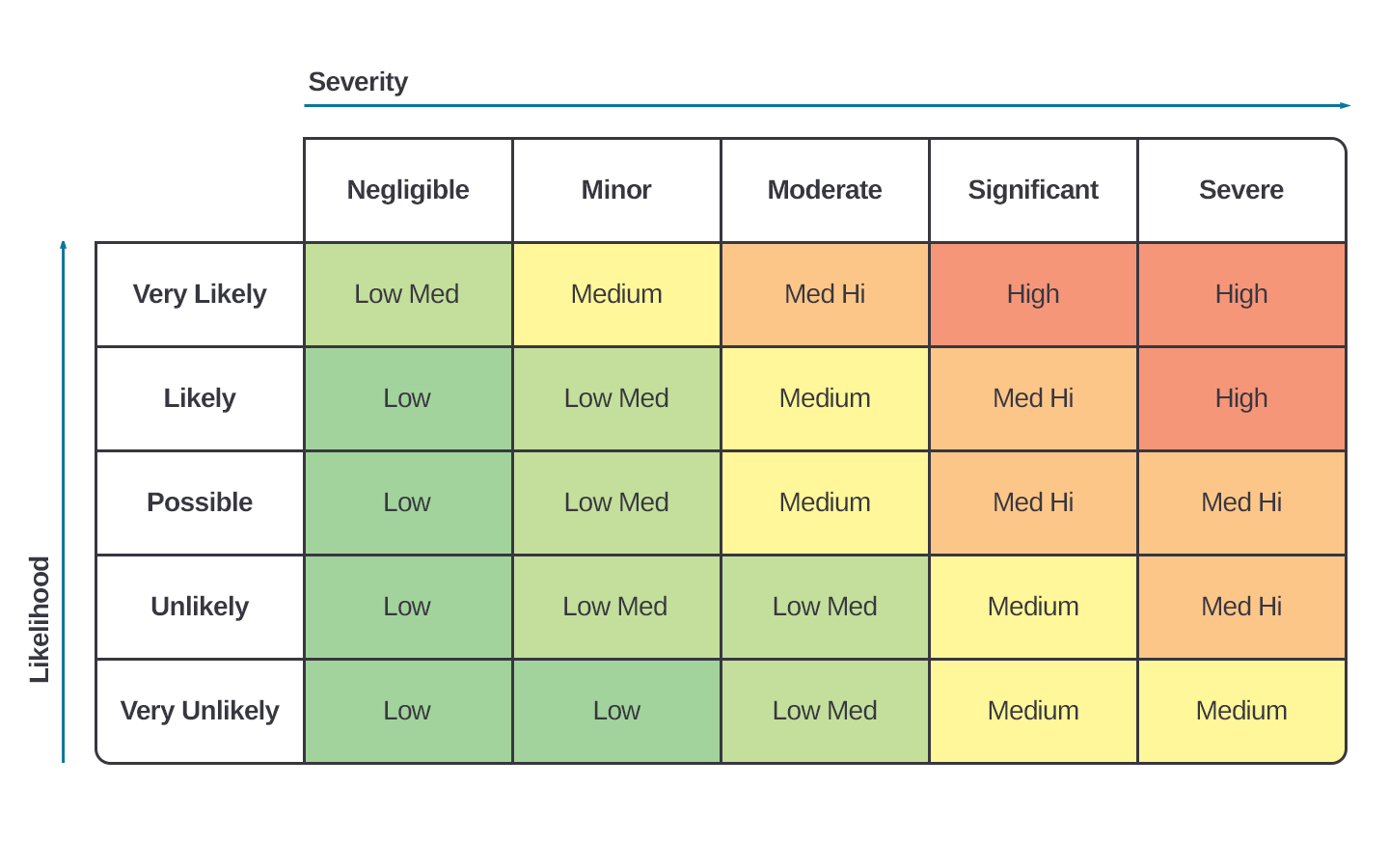
Confidently prepare for regulatory inspections and client audits with automated reviews of 100% of your quality records. Uncover gaps in GxP compliance, traceability, and documentation standards across jurisdictions. Whether you're a life sciences organization or a global supplier, our solution helps demonstrate your commitment to quality and international regulatory excellence.
Inspection/Audit readiness
Streamlined Audit Preparation for Life Sciences
Prepare seamlessly for FDA inspections and client audits with automated reviews of 100% of your quality records. Identify potential gaps in GxP compliance, traceability, and regulatory adherence. Whether you're a life sciences organization or a supplier, our solution helps you demonstrate a strong commitment to quality and regulatory standards.
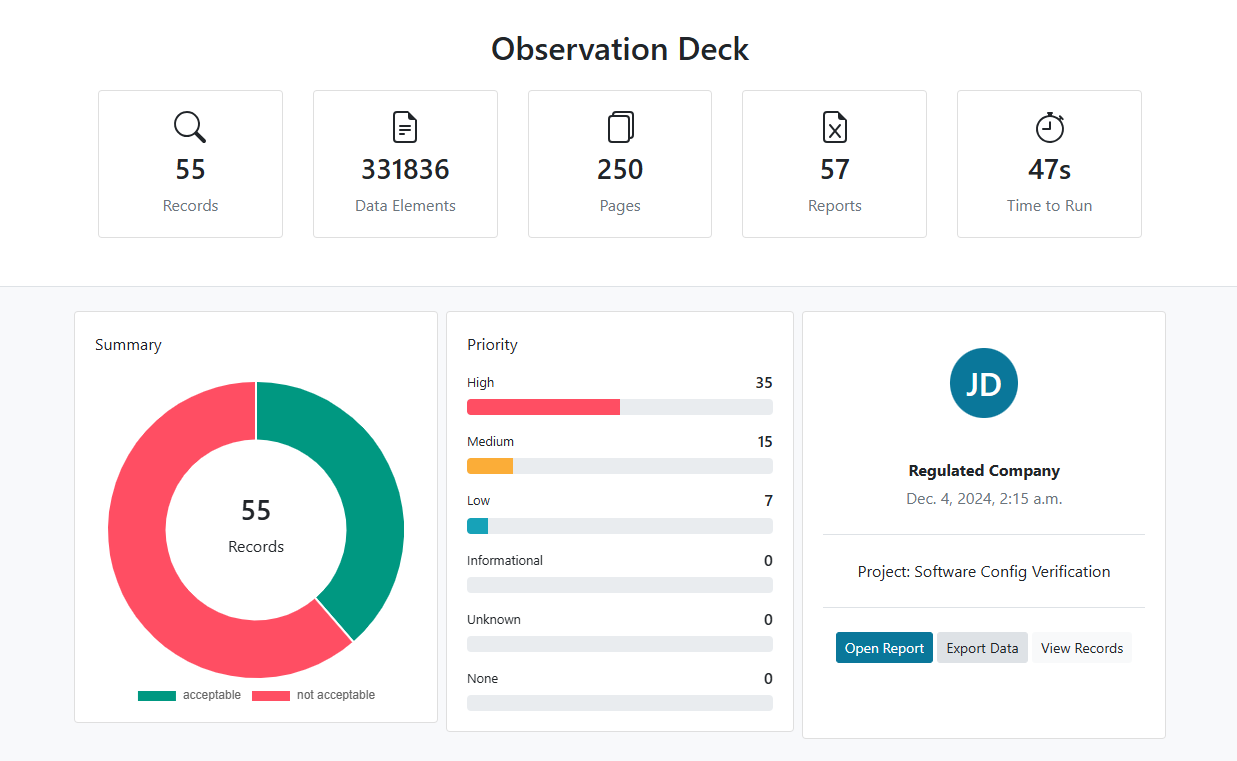
Automated Software Config Verification
Seamless Verification: Automate, Align, and Achieve Compliance
Save time and minimize errors with automated verification of software configurations against specification documents. Streamline processes to align with project requirements and meet regulatory expectations effortlessly. Reduce reliance on manual checks, freeing up resources for more strategic activities. Achieve consistent compliance while improving operational efficiency.
Automated Software Validation Review
Effortless Validation: Simplify Reviews, Focus on What Matters
Automatically review software validation reports to identify missing components and regulatory gaps. Reduce the burden of manual checks, ensuring a thorough assessment of SOP adherence. Optimize your team’s time by shifting attention to critical, high-value projects. Support compliance efforts with consistent, efficient validation processes.
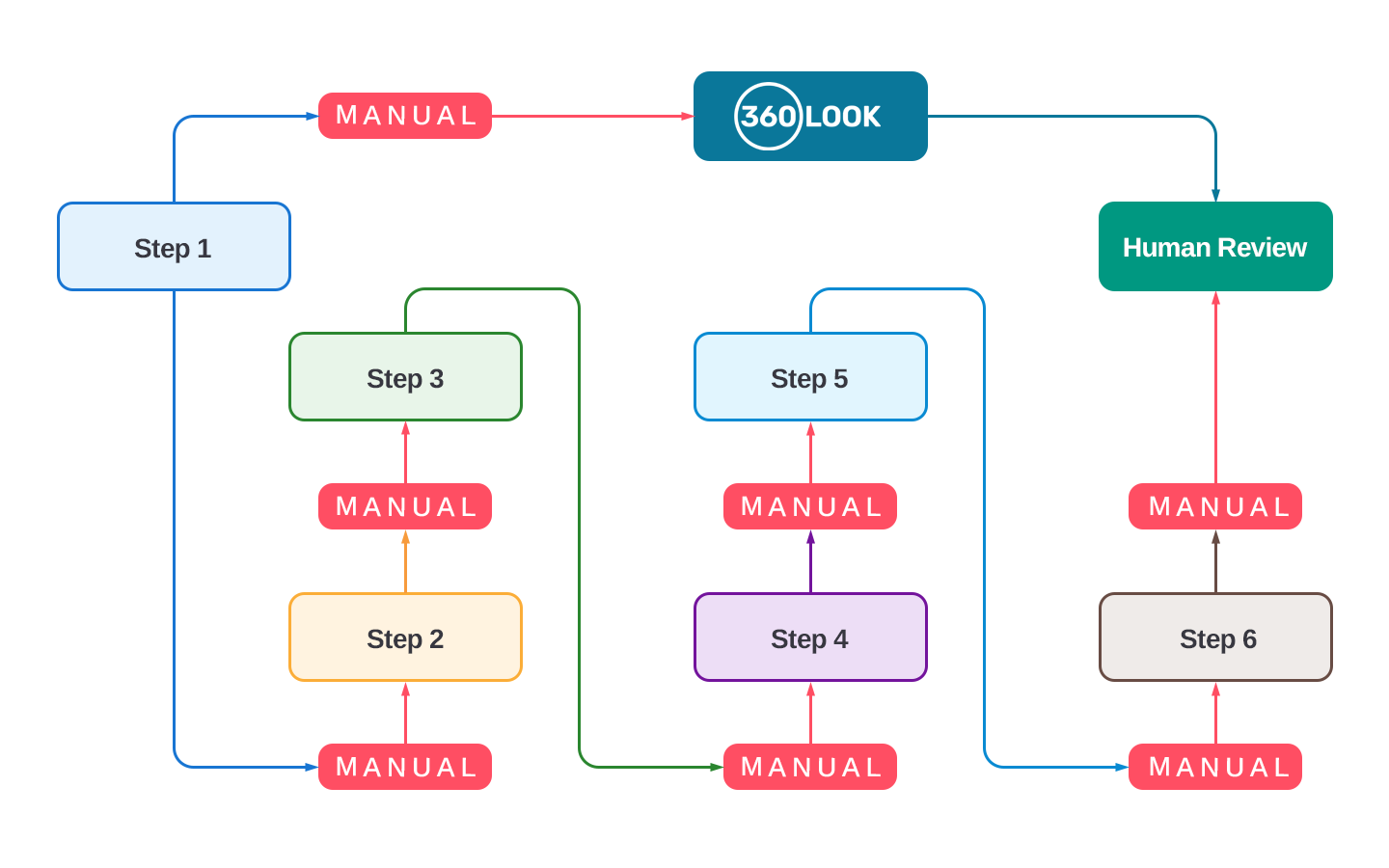
Automation of Quality Reviews
Effortless Compliance Through Automation
Reduce the burden of manual record reviews for regulatory and SOP compliance. Automate these tasks to allocate more time to supervision, training, and improving safety and quality control measures, driving greater efficiency across your organization.